top of page
Medical Device Blog
Medical Device Regulations ensure safe, effective, and innovative healthcare tools by governing their design, manufacturing, and marketing
Search
Do you want to have a CE mark? Follow the steps below
A CE mark logo is an essential identification for certain products in the EU, including medical devices and machinery.
Soledad


Regulating medical devices in the UK - April update
What you need to do to place a medical device on the Great Britain, Northern Ireland and European Union (EU) markets. This is an extract...
Soledad


Examples of Class III medical devices
Medical Device classification under MDR. Examples of MDR class III medical devices
Soledad


Examples of Class IIb medical devices
Medical device classification examples. Class IIb under MDR rules.
Soledad


Examples of Class IIa medical devices
Medical device Class IIa examples
Soledad


Examples of EU Medical Device - Class I
Examples of medical devices classes
Soledad


Impact of extension of the MDR
The European Parliament has voted to adopt an extension of the transition period for the EU Medical Device Regulations and to extend the...
pharmaserviceinc


MDR and IVDR Delay!
Today, 6th January 2023, the Commission adopted a proposal to give more time to certify medical devices to mitigate the risk of...
Soledad


EU Health Commissioner proposes MDR delay to prevent medical device shortages
The European Union Health Commissioner has proposed delaying enforcement of the Medical Devices Regulation (MDR) by three to four years
Soledad


Border line products?
#Manual on #borderline and #classification for #medicaldevices under Regulation (EU) 2017/745 on medical devices and Regulation (EU)...
Soledad


UKCA Marking - extension of standstill period
The MHRA explained its intention to extend the standstill period of using the CE Mark by 12 months to 30 June 2024 for the medical devices.
Soledad

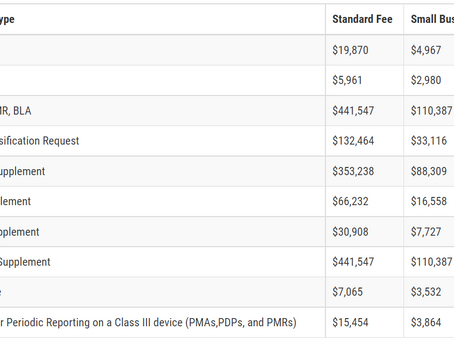
Medical Device User Fee Amendments (MDUFA)
User Fees for FY2023 Annual Establishment Registration Fee: $6,493 All establishments must pay the establishment registration fee. There...
Soledad


UPDATED GUIDANCE MHRA
This guidance document replaces the previous MHRA guidance titled “medical device standalone
software, including apps”.
-


Guidance on Classification Rules for in vitro Diagnostic Medical Devices under Regulation (EU) 2017/
Classification of in vitro diagnostic as per Regulation (EU) 2017/746
Soledad

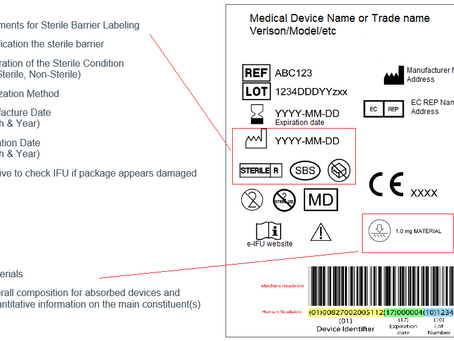
Medical Device Labelling Requirements
A label represents the written, printed or graphic information appearing either on the medical device itself, or on the packaging of each...
pharmaserviceinc


CONCLUSIONS FOR A CLINICAL EVALUATION - MDR
A successful clinical investigation for Medical Device Regulation EU 2017/745 (MDR) is one that generates scientifically valid clinical...
pharmaserviceinc


Declaration of conformity for medical devices
The Declaration of Conformity (also known as DoC) is a critical document for every medical device in Europe.
pharmaserviceinc
bottom of page

