top of page
Medical Device Blog
Medical Device Regulations ensure safe, effective, and innovative healthcare tools by governing their design, manufacturing, and marketing
Search


New AI Act Regulation
Europe's AI Act enforces transparency, safety, and accountability in AI, ensuring responsible use and protecting consumers from risks.
Soledad


UKRP (UK Responsible Person)
A UKRP (United Kingdom Responsible Person) represents non-UK medical device manufacturers, ensuring compliance with UK regulations.
Maria


Regulatory Landscape for Custom-Made Medical Devices in the EU
Navigating the complex regulatory landscape of the European Union (EU) can be overwhelming when seeking compliance, registration, and succes
pharmaserviceinc


Regulating medical devices in the UK - April update
What you need to do to place a medical device on the Great Britain, Northern Ireland and European Union (EU) markets. This is an extract...
Soledad


Examples of Class IIb medical devices
Medical device classification examples. Class IIb under MDR rules.
Soledad


Examples of Class IIa medical devices
Medical device Class IIa examples
Soledad


Examples of EU Medical Device - Class I
Examples of medical devices classes
Soledad

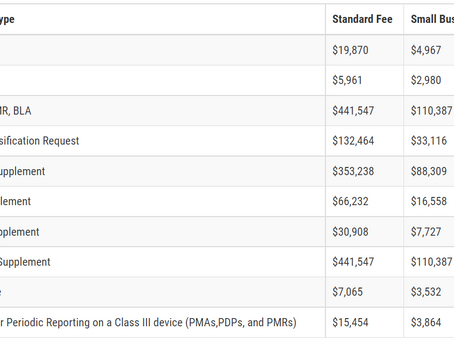
Medical Device User Fee Amendments (MDUFA)
User Fees for FY2023 Annual Establishment Registration Fee: $6,493 All establishments must pay the establishment registration fee. There...
Soledad

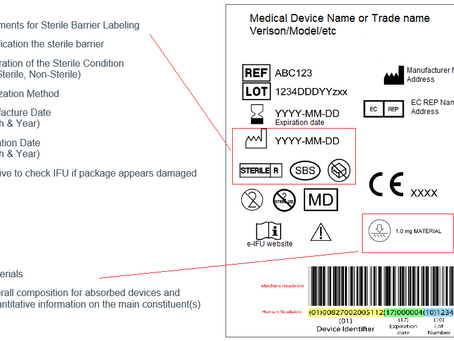
Medical Device Labelling Requirements
A label represents the written, printed or graphic information appearing either on the medical device itself, or on the packaging of each...
pharmaserviceinc


CONCLUSIONS FOR A CLINICAL EVALUATION - MDR
A successful clinical investigation for Medical Device Regulation EU 2017/745 (MDR) is one that generates scientifically valid clinical...
pharmaserviceinc


Declaration of conformity for medical devices
The Declaration of Conformity (also known as DoC) is a critical document for every medical device in Europe.
pharmaserviceinc


Are you looking for an MDR Technical File template?
MDR Technical File template
pharmaserviceinc
bottom of page

